
- Home
- All Posts
- Battery Material
- Membrane
- Application of Bipolar Membrane Eletrodialysis
Blog

Application of Bipolar Membrane Eletrodialysis
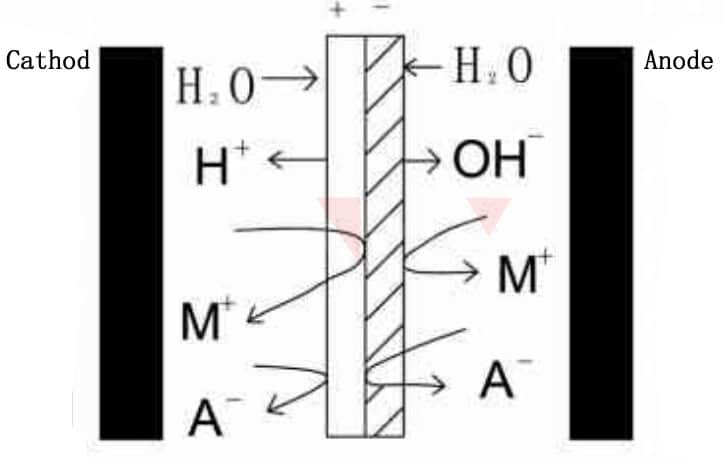 As a kind of ion exchange membrane, bipolar membrane has been industrialized since the last century. Bipolar membrane electrodialysis has been broadly used in fields such as chemical, environmental protection, food, energy supply, etc. We know that a cation exchange membrane can only allow cations to pass through under the action of direct current electric field, and an anion exchange membrane can only allow anions to pass through. The bipolar membrane has a cation exchange layer and an anion exchange layer on both sides. This special structure prevents both anions and cations from passing through the membrane layer, and can ionize water molecules(H2O) into H+and OH-. H+ migrates from anode exchange layer, while OH- migrates from the cathode exchange layer.
As a kind of ion exchange membrane, bipolar membrane has been industrialized since the last century. Bipolar membrane electrodialysis has been broadly used in fields such as chemical, environmental protection, food, energy supply, etc. We know that a cation exchange membrane can only allow cations to pass through under the action of direct current electric field, and an anion exchange membrane can only allow anions to pass through. The bipolar membrane has a cation exchange layer and an anion exchange layer on both sides. This special structure prevents both anions and cations from passing through the membrane layer, and can ionize water molecules(H2O) into H+and OH-. H+ migrates from anode exchange layer, while OH- migrates from the cathode exchange layer.
Principle of BMED technology
Bipolar membranes can form electrodialysis units simultaneously with anion/cation exchange membranes, or can form dialysis units separately with anion/cation exchange membranes.
1) Bipolar membrane+cation exchange membrane+anion exchange membrane
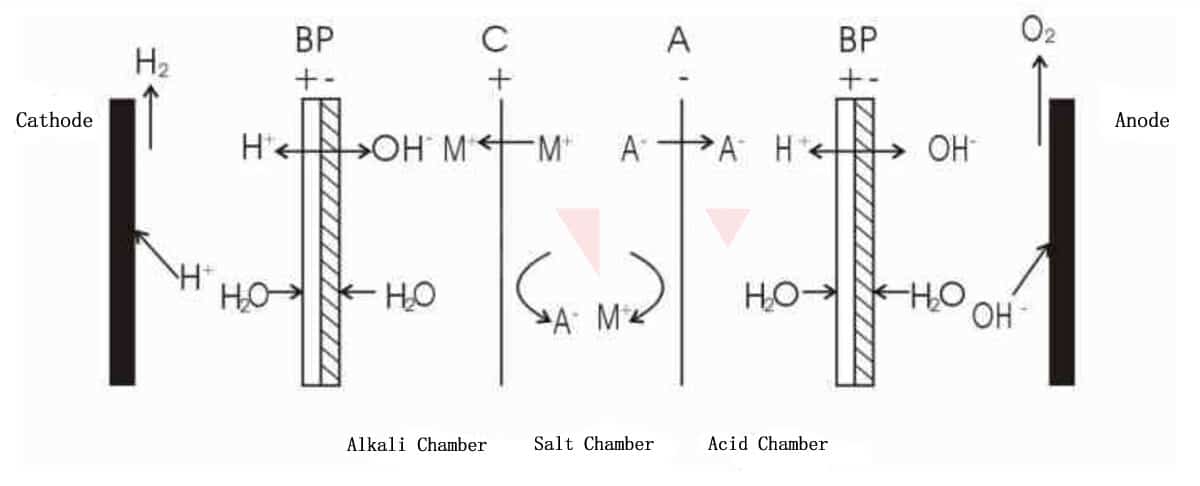
As shown in above Figure, the cation M+ in salt chamber moves towards the cathode under the action of electric field, passes through the cation exchange membrane, and combines with the OH- ionized in the alkali chamber to form an alkali. The anion A- in acid chamber moves towards the anode, passes through the anion exchange membrane, and combines with the ionized H+ in acid chamber to form an acid. As a result, the salt in salt chamber is decomposed into acid and alkali, which is suitable for the separation applications of salts of strong acids and strong bases.
2) Bipolar membrane+cation exchange membrane
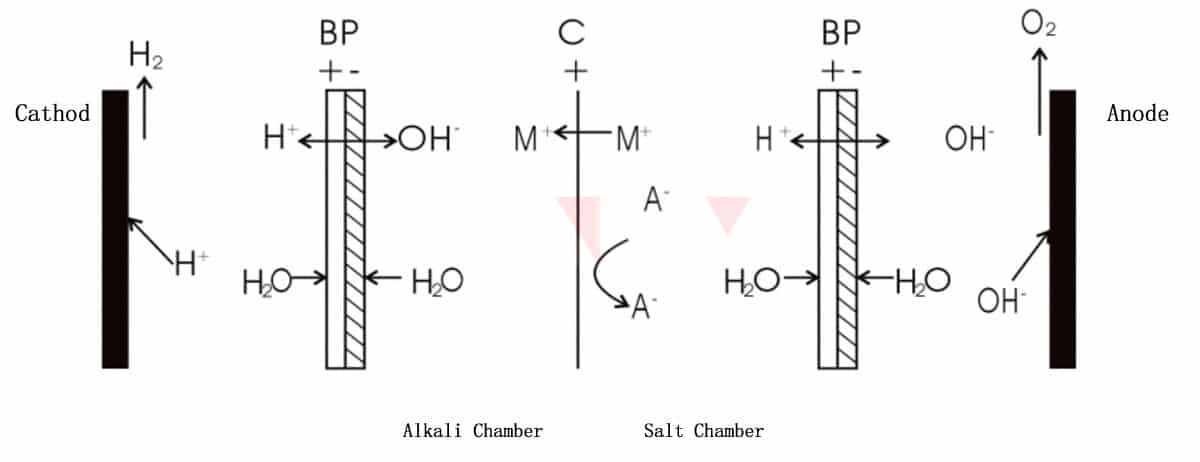
As shown in above figure, cation M+ passes through the cation exchange membrane and combines with OH- ionized in alkali chamber to form a base. The remaining anions A- and ionized H+ in salt chamber form acids. At this point, there is a mixture of salt and acid in salt chamber. This method is proper for the separation and alkali recovery applications of salts of alkali recovery of salts of strong base and weak acid.
3) Bipolar membrane+anion exchange membrane
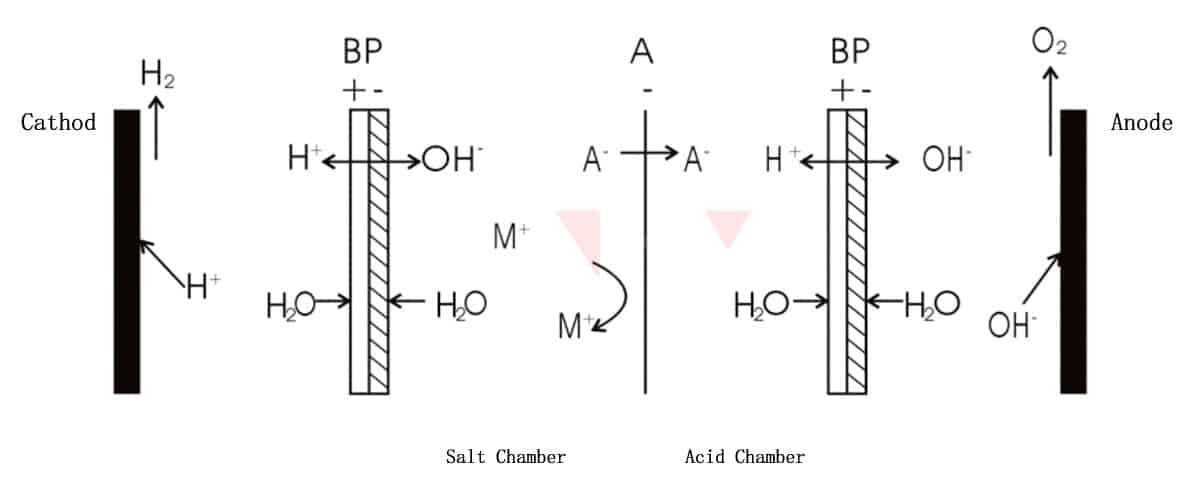
As shown in the above figure, anion A- passes through the anion exchange membrane and combines with H+ ionized in acid chamber to form an acid. The remaining cation M+ and ionized OH- in salt chamber form base. Finally, there is a mixture of salt and alkali in salt chamber. This method is appropriate for the separation and acid recovery applications of salts of strong acid and weak base.
Bipolar membrane electordialyzer
The bipolar membrane electordialyzer is composed of multiple electrodialysis units stacked together and anode and cathode electrodes at both ends. Three types of electrodialysis units can form three types of bipolar electrodialyzers:
1) Three chamber bipolar membrane electrodialysis stack. Transforming salt into acid and alkali to achieve the effect of salt removal.
2) Single positive membrane two chamber bipolar membrane electrodialysis stack. Transforming salt into a mixture of alkali, dilute salt and acid, used for separating salts of strong base and weak acid and recovering alkali.
3) Single negative membrane two chamber bipolar membrane electrodialysis stack. Transforming salt into a mixture of acid, dilute salt and alkali, used for separating salts of strong acid and weak base and recovering acids.
Compared with electrolysis, bipolar electordialyzer only produces a small amount of oxidation-reduction reaction at both poles, and the units in middle of electrode are independent, make it difficult to mix any products, and more friendly to products that are prone to oxidation-reduction reactions, besides, it avoids a large amount of electrode and energy consumption. The most important thing is to realize the recycling of acid and alkali, which not only converts waste salt back into acid and alkali, but also purifies and recovers waste alkali and acid to reduce the production costs and wastewater discharge.
Application fields of bipolar membrane eletrodialysis
1) Application in organic acid/base production. Organic acids mainly include monobasic acids, polybasic acids, amino acids with a certain water solubility, as well as thiol compounds such as thiols and sulfonic acids. Compared with the traditional calcium salt method, bipolar membrane electrodialysis doesn’t use complex lime and sulfuric acid as raw materials, only requires a certain amount of electrical energy consumption. The generated waste salt solution can be converted into acid and alkali for recycling, and the generated alkali can be recycled and reused in previous production process. Organic bases mainly include amines, hydrazine, pyridines, etc. Taking the amine synthesis process as an example, traditional methods generally involve inorganic bases in the reaction, which not only consumes caustic soda but also produces a large amount of waste salt. For water-soluble amines, the alkalization process can be achieved by removing acid radical ions through BDEM.
2) Applications in inorganic chemical industry. EDBM can reailize the conversion of water-soluble inorganic salts into inorganic acids and bases, and the recovery of water-soluble inorganic acids and bases. For example, in chlor alkali production process, alkalization is required before refining crude salt, meanwhile acidification is required before electrolysis of refined salt. Bipolar membrane electrodialysis can be used to acidify refined salt, and use produced alkali to refine crude salt, greatly reduce acid and alkali consumption and cut costs. The extraction of bromine from seawater generally involves acidification of the seawater, and then Inject chlorine gas for replacement, blow out bromine for alkaline absorption. The entire process consumes both acid and alkali. In BMED method, the alkali generated during acidification of seawater can be directly used to absorb bromine, kill two birds with one stone.
3) Application in environmental protection. Electrodialysis can select process combinations according to different water quality requirements, widely used in wastewater treatment in industries such as petrochemicals, pharmaceuticals, printing and dyeing, papermaking, electroplating, etc. BMED technology can not only effectively remove organic pollutants such as chlorobenzene, aniline and phenol from wastewater, but also remove heavy metal ions such as lead, cadmium, nickel, and further separate and recover useful substances. In the printing and dyeing industry, a large amount of wastewater is generated from manufacturing process including desizing, bleaching, cooking, dyeing and printing, etc. EDBM can be used to recover dyes from liquid waste, and Put the recovered acid and alkali into recycling to save water resources and avoid sewage discharge. During the papermaking process, it is necessary to chemically cook plant fibers, resulting in a large amount of waste liquid (commonly referred to as black liquor). Due to the differences in components between raw materials and cooking agents, the composition of black liquor is very complex. The conventional approach is to extract organic compounds from black liquor as chemical raw materials. The extracted inorganic salt solution can be recovered by bipolar membrane electrodialysis to recover alkali and acid, efficiently complete the comprehensive utilization of wastewater.
About Mr. Zhou
Search
Recent Posts
-
Manufacturing Process of Ca... 11/28/2024
-
Application of Flexible Gra... 05/14/2024
-
PEM Water Electrolysis for ... 04/12/2024
-
Application of Bipolar Memb... 01/09/2024
-
Membrane Electrode Assembly... 11/27/2023
Categories
- All Posts (24)
- Flow Battery (11)
- Battery Material (20)
- Bipolar Plate (13)
- Membrane (3)
- Felt Electrode (1)
- MEA (3)
- Fuel Cell (5)
Contact Info.
Recent Post
-
Manufacturing Process of Ca... 11/28/2024
-
Application of Flexible Gra... 05/14/2024
-
PEM Water Electrolysis for ... 04/12/2024
-
Application of Bipolar Memb... 01/09/2024
-
Membrane Electrode Assembly... 11/27/2023


