
- Home
- All Posts
- Flow Battery
- Vanadium Redox Flow Battery
- PFSA Membranes For VRFB
Blog
PFSA Membranes For VRFB
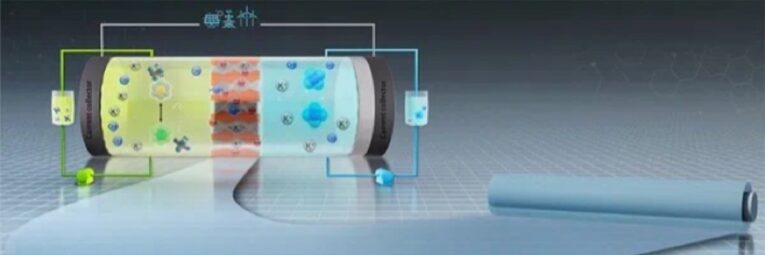
As an aqueous redox flow batteries, Vanadium Redox Flow Batteries (VRFB) are one of the most widely applied in electrochemical energy storage systems for the large-scale storage applications. As a key component of VRFB, Ion exchange membrane plays a critical role on flow battery performance. PFSA membranes made by XNHY, are based on PFSA polymer, which have excellent mechanical properties, good ionic conductivity and chemical stability, vanadium battery, widely used in all vanadium flow battery industry.
Types of ion exchange membranes for vanadium batteries
According to active groups, ion exchange membrane for VRFB can be divided into cation exchange membrane and anion exchange membrane. The common Nafion membrane from DuPont belongs to cation exchange membrane, and common SEI membrane belongs to anion exchange membrane, has high efficiency but high price and short service life. According to content of fluoride, it can be divided into perfluorosulfonic acid membrane, semi fluorosulfonic acid membrane and non fluorosulfonic acid membrane. Perfluorosulfonic acid(PFSA) membrane, which has been widely used in chlor alkali industry, is generally used for Vanadium redox flow batteries due to its stability and long service life.
Requirements of proton exchange membrane for VRFB
1) Physical and chemical properties. Including the mechanical strength of the membrane material, corrosion resistance and oxidation resistance to Vanadium electrolyte, which has an important impact on the cycle life.
2) Ion exchange capacity. Ion exchange capacity(IEC) is an important index. Generally, the higher the IEC is, the higher conductivity of ion exchange membrane is. In addition, the membrane surface resistance is also an important index for conductivity, directly affects the internal resistance of VRFB. Higher ion permeability means lower membrane resistance, which are beneficial to the efficiency and performance optimization of vanadium battery.
3) Lower vanadium ion transmittance. Vanadium resistance is the ability that ion exchange membrane prevents the penetration of vanadium ions in the membrane. Vanadium resistance greatly determines the current efficiency of VRFB. In addition, the adsorption of the ion exchange membrane on the electrolyte also reflects the barrier ability of proton exchange membrane to vanadium ions. Less adsorption means the better the vanadium resistance performance, which is conducive to the assembly and stable operation of Vanadium redox flow battery.
4) Optimal thickness. If the thickness of the exchange membrane is too thin, the mechanical properties and vanadium resistance of exchange membrane will be reduced, and further affect long-term and stable operation of flow battery. On the contrary, the battery stack assembled with a thicker exchange membrane will increase the internal resistance of battery, result in the loss of voltage efficiency and electric energy. The uniform thickness of membrane also leads to the uniform current passing through the membrane surface. Therefore, it is very important to choose an ion exchange membrane with uniform and appropriate thickness.
Application of PFSA Membranes For Vanadium Redox Flow Batteries
At present, many ion-exchange membranes used for water treatment in the market are not suitable for development of vanadium batteries due to their high resistance or poor chemical stability. Perfluorosulfonic acid(PFSA) membrane has become the most widely used ion exchange membrane and has been accepted by vanadium battery manufacturers at home and abroad. Nafion membrane of DuPont with high permeability of vanadium ions and high price, greatly limits the commercialization and popularization of VRFB.
The high vanadium barrier exchange membrane has been developed by XNHY specially for vanadium batteries, which is made of high-quality perfluorosulfonic acid(PFSA) ion-exchange resin, together with a new casting process. Vanadium barrier resin material is added in the casting process, which greatly reduces the permeability of vanadium while maintaining a small decrease in conductivity. PFSA membranes possesses high strength, high conductivity and stable chemical performance, and broadly used in the fields of VRFB and other redox flow batteries, fuel cells and so on.
About Mr. Zhou
Search
Recent Posts
-
Manufacturing Process of Ca... 11/28/2024
-
Application of Flexible Gra... 05/14/2024
-
PEM Water Electrolysis for ... 04/12/2024
-
Application of Bipolar Memb... 01/09/2024
-
Membrane Electrode Assembly... 11/27/2023
Categories
- All Posts (24)
- Flow Battery (11)
- Battery Material (20)
- Bipolar Plate (13)
- Membrane (3)
- Felt Electrode (1)
- MEA (3)
- Fuel Cell (5)
Contact Info.
Recent Post
-
Manufacturing Process of Ca... 11/28/2024
-
Application of Flexible Gra... 05/14/2024
-
PEM Water Electrolysis for ... 04/12/2024
-
Application of Bipolar Memb... 01/09/2024
-
Membrane Electrode Assembly... 11/27/2023


